Feynman may have been wrong about Brownian ratchets
A Brownian ratchet is any device that can harvest energy from Brownian motion, that is, random thermal motion such as the motion of air particles in a room. In the 1960s, Richard Feynman, the famous Nobel Laureate and bongo player, argued that Brownian ratchets were impossible, and for 60 years, no one questioned that argument.
After all, doing useful work requires some differential, a transfer from hot to cold, or high to low. Surely, when everything is in thermal equilibrium, even if there is energy there in the form of heat, that energy is static. It cannot do work.
Yet, a team in Arkansas has slowly been working to get around the laws of thermodynamics and extract small amounts of energy from ambient temperatures using the thermal properties of a relatively new substance: graphene. The response from the scientific community has been muted so far, but funding sources and the media are interested. Can we produce power from essentially nothing but jiggling molecules? Was Feynman wrong?
The first law of thermodynamics, indeed, says that the change in the energy of a system is the heat added minus the work done.
The second law, meanwhile, says that for a closed system, entropy must always increase.
The second law was derived from Sadi Carnot’s studies of steam engines, a good example of when technology precedes science. Carnot wanted to understand how these engines made things go. At the time, the dominant theory for how such engines worked was called the caloric theory. This theory proposed that heat was a fluid called caloric. Engines consume caloric in order to produce movement.
The 18th-century scientist Lavoisier promoted the caloric theory as a means to explain why fluids change volume when heated or cooled. He argued that a “subtle fluid” filled the spaces between the molecules when the fluid was heated.
Much like the aether of the late 19th century that Einstein disproved, this theory proposed the existence of a fluid where none was required. Molecules move apart when heated, generally because they are moving faster and have more entropy. (Caloric theory also doesn’t make a lot of sense when you consider that water expands when it is frozen, although substances do get wonky across phase transitions.)
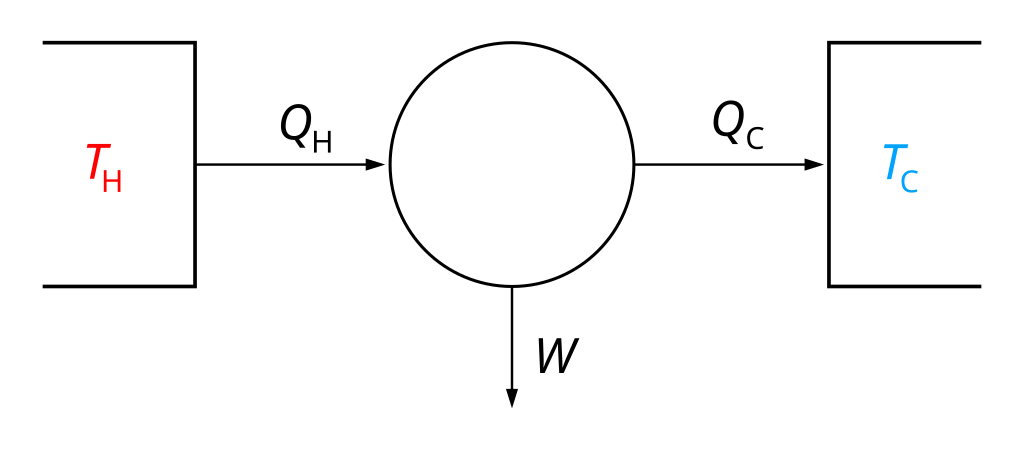
Carnot, nevertheless, made a huge impact on the future of science when he invented a thought experiment, the Carnot engine. This theoretical engine consists of two reservoirs of heat, which he thought of as containing different amounts of caloric. The first reservoir, H, he called the furnace, and the second, C, he called the refrigerator. Both are kept at constant temperature even as energy transfers from H to C. When energy transfers from H to C, it can do some mechanical work as well, which explains why steam engines, using a boiler kept, can convert that heat into locomotive power by transferring some heat energy, in the form of steam, to a cylinder which has been cooled by mechanical expansion. Internal combustion engines work much the same way.
Carnot’s engine operates by something now called the Carnot cycle, which shows that the work done by such an engine relates to the temperature difference times the ratio of heat transferred and the hot reservoir temperature. (Which means that indeed, a hotter reservoir needs to transfer more energy per cycle to do the same amount of work, and a colder refrigerator reservoir means more work is done.)
Carnot’s engine can also be reversed. Mechanical work can transfer energy from a cold reservoir to a hot one. This is how most air conditioners and refrigerators work, using a mechanical compressor to transfer energy, via a refrigerant, to the surrounding air.
Prussian scientist Rudolf Clausius, among others in the 19th century, used Carnot’s theory to derive a form of what we now call the second law of thermodynamics:
Heat can never pass from a colder to a warmer body without some other change, connected therewith, occurring at the same time. (Clausius, 1854)
This seems obvious. Heat passes from hot to cold, never in reverse, unless there is something else, like a compressor, forcing the change.
This also means that if two regions are at the same temperature, then no work can be done by the flow of energy from one to the other.
Now, we know that there is no such thing as “heat” in the sense of a fluid like caloric. Heat is simply a property that molecules carry. Indeed, when you have a small number of molecules, it is impossible to say how much heat they carry. They have energy, for sure, but concepts like temperature, pressure, and so on that we typically want to quantify when we talk about "heat” are ill-defined with small sample sizes.
All this became mathematically precise with the work of Ludwig Boltzmann, Gibbs, and others who developed statistical mechanics in the late 19th century, building on thermodynamics. Statistical mechanics connected the Newtonian mechanics of individual particles or molecules of gases and fluids to thermodynamic concepts like temperature, entropy, pressure, specific heat, and so on.
This theory showed that thermodynamics was the result of the law of large numbers. The more molecules you had, the more true the laws were. Since most everyday applications involve trillions of trillions of molecules, these laws are very true.
But with that development, it seemed like there was an opening to defy the so-called “laws” of thermodynamics. If they were, after all, only statistical and not absolute, surely, they could be violated at small scales?
Enter the Brownian Ratchet.
Keep reading with a 7-day free trial
Subscribe to The Infinite Universe to keep reading this post and get 7 days of free access to the full post archives.